Our story
Bertrand Gabry, Bill Muirhead and Charles Fontannaz worked extensively together for Valtronic Technologies.
The three friends and work colleagues had the ambition to use their complementary experience and skill-sets to create a global Business Consulting firm able to bring entreprising solutions adapted to the needs of regional, national and international entrepreneurs.
In 2011, Bill Muirhead and Charles Fontannaz, created VFM Conseil SA providing strategic consulting, audit and accounting & tax services.
In 2014, GMB Services SA was created to bring Engineering , Quality Assurance & Regulatory Affairs and Operational Excellence services, particularly to clients in the medtech and industrial sector.
We help you to concentrate on what is important for the development of your business
"Some people want it to happen, some wish it would happen, others make it happen"
Michael Jordan
our approach
To make a great product, deliver a great service and build long-term business, you need to have the right vision, the right ingredients and the right people.
To succeed you need to be passionate and patient (not always…!).
To really understand our clients’ business and needs.
To challenge existing ideas to bring innovative solutions for our clients.
To work with entrepreneurs who are change-orientated, who are prepared to challenge the norm, to help them make transformative decisions to deliver success.
Our values
We are entrepreneurs, like our clients, who strive to be innovators, never satisfied with the status quo.
We are passionate about what we do and building long-term relationships with our clients.
We have a commitment to the highest quality, professionalism and excellence in everything we do.
We strongly believe in ethical standards in everything we do.
We are not afraid to say what we think. Tell it like it is in a direct and straight-forward manner.
Our DNA
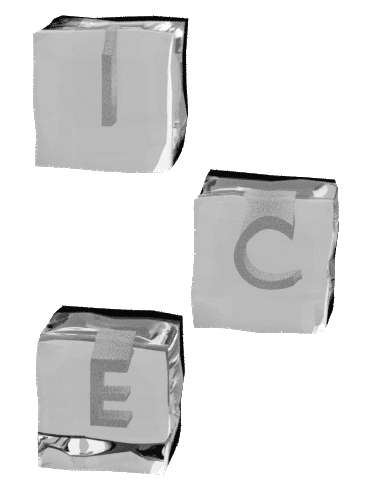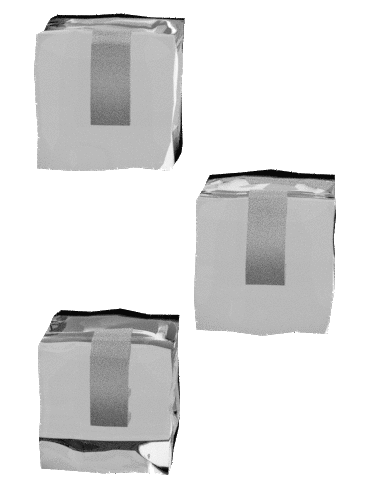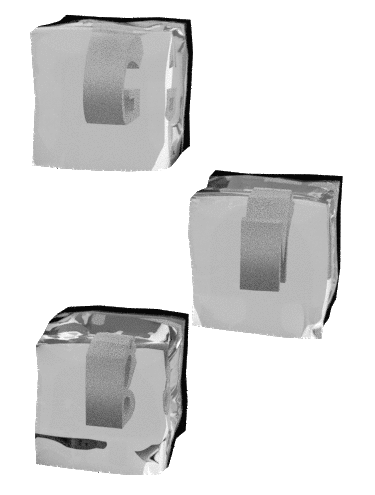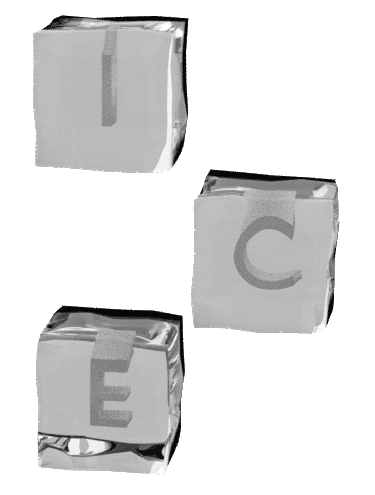
I nnovation ntegrity nvolvement nternational
C ompliance onfidence hallenge ompetitive
E fficiency thic nvironment xpertise
Our team
Our experts who provide engineering and consulting solutions.

BERTRAND GABRY
CEO / Senior Consultant
Senior Engineering, QA/RA & Business Excellence Expert
Bertrand Gabry has a Materials Science and Engineering PhD from the University of Franche-Comté and a degree in Mechanics and Production from the University of Bourgogne.
He is a team leader with extensive experience in his field, the ability to assess the operational organization, and the ability to provide pragmatic and effective solutions to complex problems.
Previously, he was responsible for the Spine Business Unit, Group Quality & RA Manager of Valtronic Technologies, Quality Manager of AP Technologies and the Pneumatics Division of Parker Hannifin.
Bertrand has experience in the automotive, industrial, medical technology and luxury sectors.
He has expertise in management, engineering, project management and QA/RA.
He is also a Board member of a number of different companies.
Key Figures

10 Partners

14 QMS implementation
(ISO13485 – ISO9001 – ISO14001)
24 Regulatory Inspections (Brazil, China, FDA, South Korea, EU)

50 Years of cumulated experiences in Medtech (Class 1 to Class 3)

55 Customers

237 Quality audits (internal, external)

12’800 h Engineering project

